Researchers Are Developing a New Screening Tool for Upper GI Tract Diseases
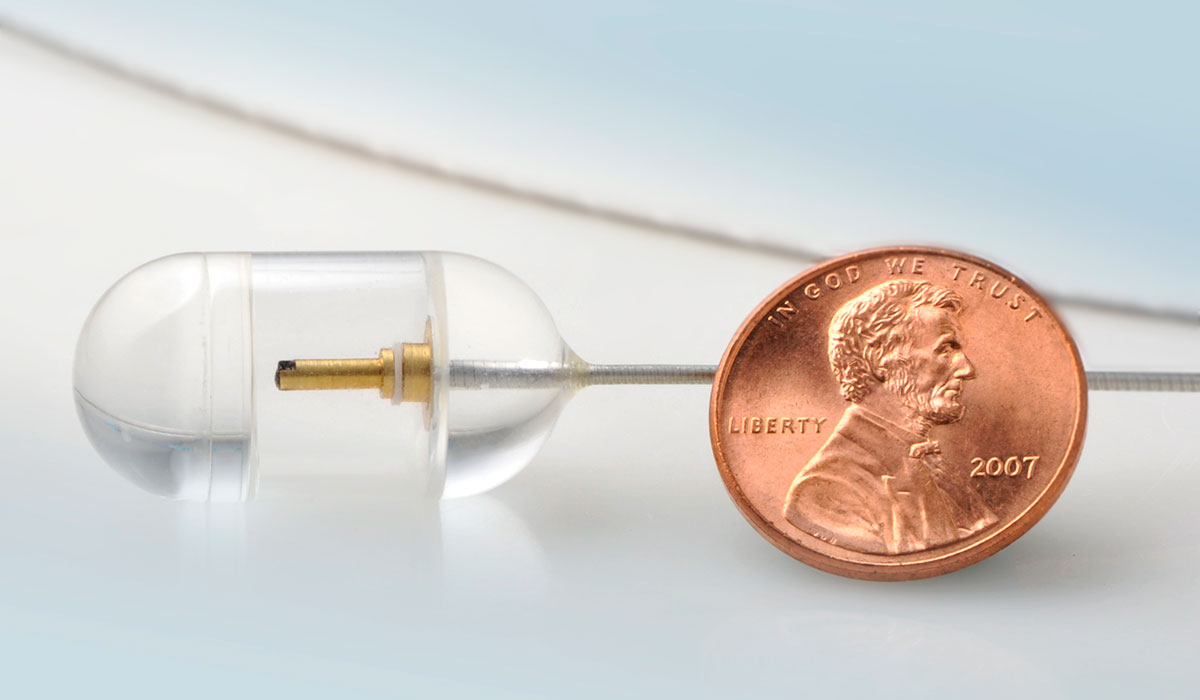
the pill-shaped device captures high-resolution images of the esophagus to help physicians screen for and diagnose various diseases. photo provided to bostonmagazine.com.
For many people, undergoing an endoscopy can be both a costly and physically uncomfortable experience. Now, thanks to new technology developed by researchers at Mass General, those challenges may soon be a thing of the past.
Typically performed by a specialist, a traditional endoscopy involves the insertion of a tube with a camera down a patient’s throat to expose the lining of the upper gastrointestinal (GI) tract. The procedure helps physicians screen for and diagnose a number of diseases including Barrett’s Esophagus, which—according to the Mayo Clinic—is a precancerous condition characterized by frequent heartburn and difficulty swallowing.
The new technology, known as tethered capsule endomicroscopy, achieves the same aims by using a pill-shaped device that is swallowed by a patient. The device, roughly the size of a large vitamin, is designed to capture three-dimensional, microscopic images as it travels down the esophagus toward the stomach. It’s attached to a tether that doctors use to control the capsule’s speed and position after it’s swallowed, as well as to remove it at the end of the procedure.
Gary Tearney, a pathologist at Mass General and associate director of the hospital’s Wellman Center for Photomedicine, says that the new technology is less invasive than traditional endoscopy and won’t require sedation. He also says that it will cost less than the traditional procedure because the capsule can be reused after it’s disinfected; patients won’t have to take as much time off from work; and the procedure—which only takes a few minutes—won’t need to be performed by a specialist. Currently, researchers are also exploring the use of the capsule in primary care settings.
“The idea is that you’d go into the primary care office, you’d get your blood pressure taken, you would be weighed, and then you’d quickly swallow this capsule and that would constitute your early phase screening even before you see your physician,” Tearney says. “That’s a really powerful paradigm.”
Though tethered capsule endomicroscopy is still in the development and testing stages, Tearney says he expects that the technology will be available within the next couple of years. He says researchers have designed five or six different versions of the device at this point, and are conducting clinical trials to evaluate patient tolerance and imaging quality. Later studies will focus on using the capsule to monitor the evolution of Barrett’s Esophagus over time, which, he says, “will provide a lot of really important information [about] how to best manage the disease.”
For now, Tearney says that he is excited by the capsule’s potential to change the model for screening for upper GI tract diseases. “We’ve heard a lot of feedback from people who have had endoscopies that they’ve disliked the procedure so much that they recommend to their friends that they not do an endoscopy even if they need one. I think the perception about [the procedure] also affects how many people get endoscopy,” he says. “My hope is that we can completely change the paradigm for screening for upper GI tract diseases and make it commonplace, make it cost effective, and really start to decrease the mortality of some of these cancers.”


