Researchers Develop ‘Trauma Foam’ to Combat Internal Bleeding
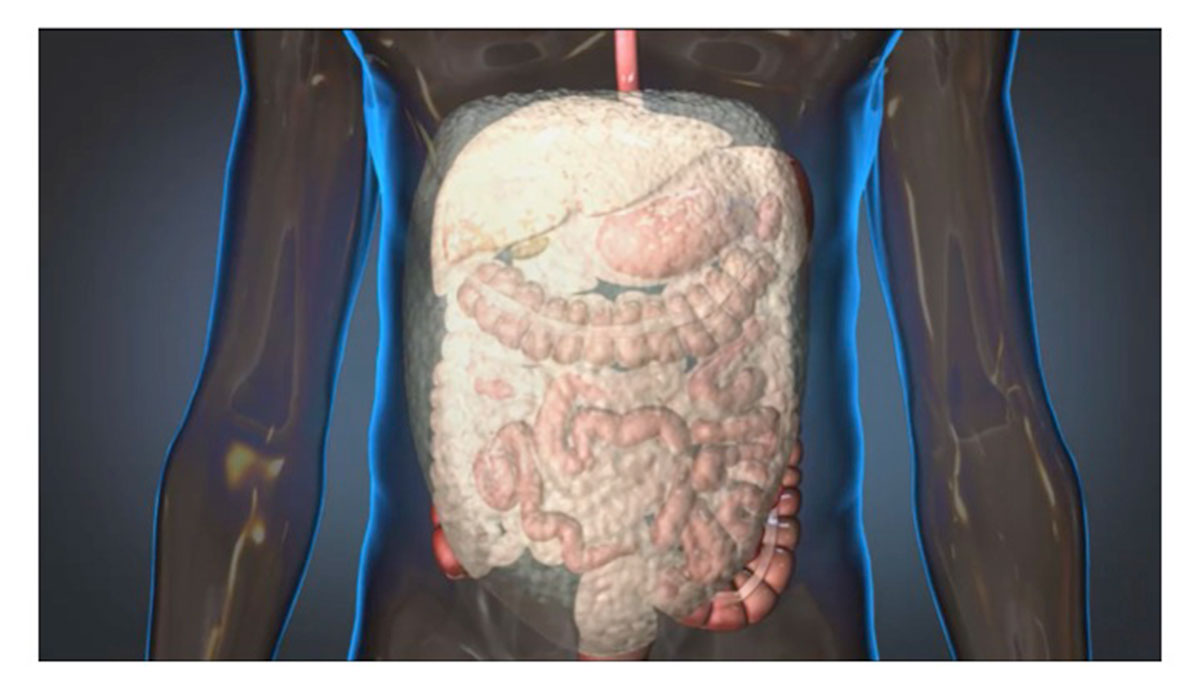
A chemical reaction causes the foam to expand and apply the pressure needed to slow internal bleeding. Photo provided to bostonmagazine.com by arsenal medical.
As a trauma surgeon and veteran of two wars, David King has seen his fair share of fatal injuries. But none have been more devastating than those he could have prevented.
“Having been in both Iraq and Afghanistan, one of the most heart wrenching situations for me to be in when I’m deployed is for someone to bring me a fellow soldier, and for me to determine that they have no vital signs and that they bled to death somewhere between the time they got injured and the time they got to me,” says King, now an attending physician at Massachusetts General Hospital. “The number of soldiers that I’ve had to declare dead because they come to me with injuries that likely I could have fixed had they shown up a little sooner with some signs of life hits very close to home.”
That’s why King has teamed up with Watertown-based company Arsenal Medical and other researchers at Oregon Health Sciences University and the University of Texas-Houston to develop a new “trauma foam,” a first-of-its kind technology designed to slow the rate of internal bleeding long enough for a patient to reach a hospital and receive a life-saving operation.
The foam is injected into a patient’s abdomen in two phases, resulting in a chemical reaction that causes the foam to expand and apply the necessary pressure to temporarily quell the internal bleeding. According to animal studies conducted by King and his collaborators, the foam can potentially extend a patient’s life by as many as three hours—a significant amount of time considering that injuries of this kind can become fatal in a matter of minutes, King says.
Researchers have recently completed a new study of the foam using human cadavers, a novel technique that allowed them to determine the optimal foam dosage to administer to live patients without compromising their safety. The findings of this unique study were presented earlier this month at the 28th Eastern Association for the Surgery of Trauma Annual Scientific Assembly. The next step in the process is to acquire FDA approval, which King says he hopes—and expects—to be granted later this year.
In addition to its obvious usefulness on the battlefield, researchers say the foam, which has been in development for more than four years, could be a valuable treatment option for civilian patients as well, particularly those who are injured in car crashes or other serious accidents.
“Following FDA approval, this will be the first therapy ever that could potentially be used in the pre-hospital environment to rescue patients from bleeding to death and allow them to arrive at a hospital so a surgeon can operate on them,” King says.
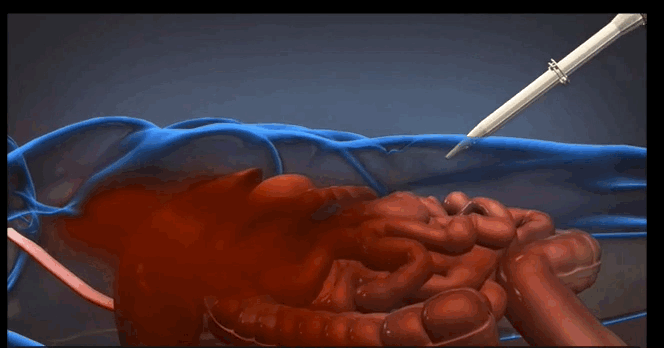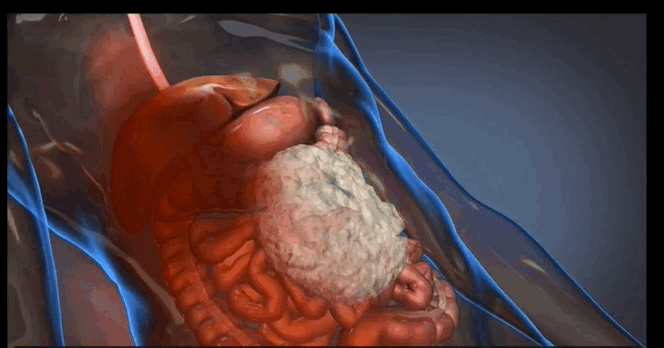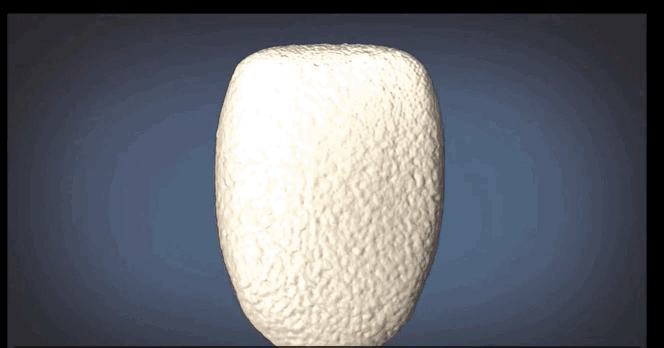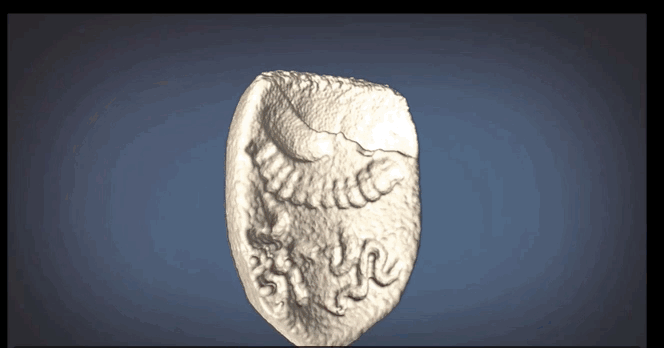
For more information about the trauma foam and how it works, check out this brief video clip at arsenalmedical.com.


